by Buck Institute
March 29, 2021 . News
Chronic viral infections can have lasting effects on human immunity, similar to aging
Research from the Buck Institute and Stanford University suggests that chronic viral infections have a profound and lasting impact on the human immune system in ways that are similar to those seen during aging. Results are published in Proceedings of the National Academy of Sciences.
Using systems immunology and artificial intelligence, researchers profiled and compared immune responses in a cohort of aging individuals, people with HIV on long-term anti-retroviral therapy, and people infected with hepatitis C (HCV) before and after the virus was treated with a drug that has up to a 97% cure rate. Shared alterations in the immune system include T cell memory inflation, upregulation of intracellular signaling pathways of inflammation, and diminished sensitivity to cytokines in lymphocytes and myeloid cells.
 “Chronic inflammation stemming from immune system dysfunction is associated with many of the diseases of aging,” says David Furman, PhD, Buck Institute associate professor and senior author of the paper. “Whether chronic viral infection contributes to age-associated immune dysfunction is still an open question, but studies of this type provide a way to start getting answers. At this point it’s clear that both aging and chronic viral infections leave profound and indelible marks on immunity.”
“Chronic inflammation stemming from immune system dysfunction is associated with many of the diseases of aging,” says David Furman, PhD, Buck Institute associate professor and senior author of the paper. “Whether chronic viral infection contributes to age-associated immune dysfunction is still an open question, but studies of this type provide a way to start getting answers. At this point it’s clear that both aging and chronic viral infections leave profound and indelible marks on immunity.”
The difference between acute and chronic viral infections
In acute viral infections the body is usually able to clear the offending agent and the immune system (in the best-case scenario) produces antibodies that protect against similar infections – think of common colds and seasonal flus. But there are viruses, in addition to HIV and HCV, which can remain alive, setting up “host-parasite housekeeping” in the body, in some cases without people being aware of them. Furman says depending on geographic location, 70 to 90% of the population is infected with cytomegalovirus, which is harmless in healthy individuals and is only problematic for pregnant women or those with compromised immune systems. Various herpes viruses (which cause genital herpes, cold sores, chicken pox/shingles, and mononucleosis) can also lead to chronic infections.
“Each of us has our own virome; it’s the collection of the viral infections you have during your lifespan,” Furman says. “You probably have been infected by 12 or 15, or even more viruses that you never knew you had. Fortunately technology now exists that allows us to profile these infections in the human population; it is helping us move these types of inquiries forward.” Furman says this study is the first to fully incorporate the concept of systems immunology and holistically analyze the immune system using the same technological platforms across different cohorts of patients.
Reason for hope – results show some plasticity in immune response
The study showed that in patients with HIV, immune system dysregulations were evident despite having been treated with virus-suppressing drugs for over ten years. But clearance of the HCV virus (via the drug sofosbuvir) partially restored cellular sensitivity to interferon-a, which inhibits viral replication. “This plasticity means there is room for intervention in both chronic viral infections and in aging,” says Furman. “It’s just a matter of identifying and understanding the molecular pathways and networks involved.” This paper identified changes in STAT1, the primary transcription factor activated by interferons. STAT1 plays a major role in normal immune responses, particularly to viral, mycobacterial and fungal pathogens.
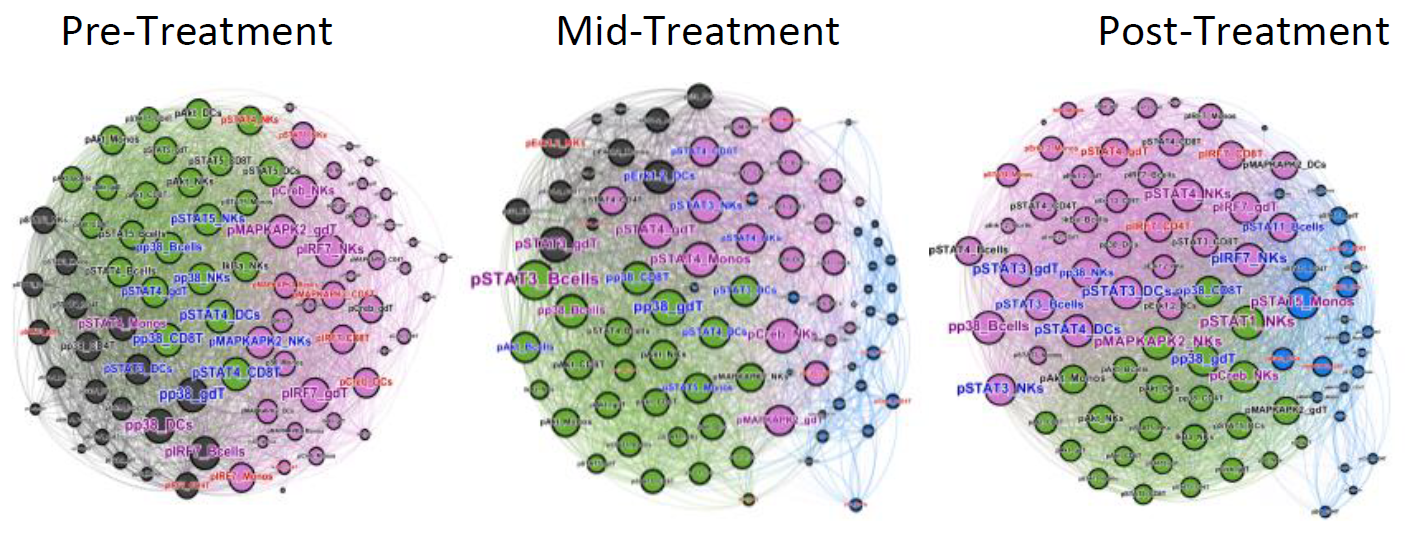
Network topology analysis of immune system function representing dozens of integrated cellular responses that are rewired during removal of the hepatitis C virus in humans. Different communities (functional clusters, colored) are determined by the signaling pathways that are being interrogated. For example the black community observed during active HCV infection is largely configured by MAPK activity; shrinks with the decrease in viremia, and disappears after complete viral clearance. A nascent blue population mid-treatment configured by proliferation/survival/differentiation (P/S/D)/STAT1 signaling activities (largely anti-viral) expands post sofosbuvir treatment.
Implications for COVID-19?
Furman says we are in the midst of an ongoing “living” experiment in regards to the COVID-19 pandemic. He says future studies are needed to determine whether the functional imprinting of the immune system is hardwired to only involve the chronic nature of specific infections, or whether relatively short-lived but vigorous inflammations such as COVID-19 also leave a long-lasting footprint on the immune system. “Has the immune system of those infected with the coronavirus taken a big hit? That’s a theory, but we don’t know what will happen,” says Furman, who is collaborating with Stanford University and the University of California, San Francisco on projects involving COVID-19 and immunity.
Citation: Signatures of immune dysfunction in HIV and HCV infection share features with chronic inflammation in aging and persist after viral reduction or elimination
Other Buck Institute collaborators include Huixan Du and Kevin Perez. Additional co-authors include Cesar J. Lopez Angel, Edward A. Pham, Benjamin J. Fram, Thai Nguyen, Yael Rosenberg-Hassan, Holden T. Maecker, Jeffrey S. Glenn, and Mark M. Davis, Department of Microbiology and Immunology, Stanford University School of Medicine, Palo Alto, CA; Francesco Vallania, Institute for Immunity, Transplantation and Infection, Stanford University School of Medicine; Aijaz Ahmed, Division of Gastroenterology and Hepatology, Stanford University School of Medicine; Purvesh Khatri, Stanford Center for Biomedical Informatics Research, Stanford University School of Medicine; Cornelia L. Dekker, Department of Pediatrics, Division of Infectious Diseases, Stanford University School of Medicine; and Philip M. Grant, Division of Infectious Diseases, Department of Medicine, Stanford University School of Medicine.
Science is showing that while chronological aging is inevitable, biological aging is malleable. There's a part of it that you can fight, and we are getting closer and closer to winning that fight.
Eric Verdin, MD, Buck Institute President and CEO