by Buck Institute
September 24, 2019 . Press Release
Cellular senescence is associated with age-related blood clots
Cells that become senescent irrevocably stop dividing under stress, spewing out a mix of inflammatory proteins that lead to chronic inflammation as more and more of the cells accumulate over time. Publishing in the September 24 edition of Cell Reports, researchers at the Buck Institute identified 44 specific senescence-associated proteins that are involved in blood clotting, marking the first time that cellular senescence has been associated with age-related blood clots.
“The incidence of venous thrombosis, which includes deep vein thrombosis and pulmonary embolism is extremely low until the age of 45, when it begins to rise rapidly. Over time it becomes a major risk factor for death. By 80, the condition affects five to six people per thousand individuals,” said Judith Campisi, PhD, Buck professor and senior co-author of the study. “Blood clots are also a serious side effect of chemotherapy, which sets off a cascade of senescence in those undergoing treatment. That’s why blood thinners, which carry their own risks, are often included in treatment protocols.”
Scientists in the Campisi lab and other labs around the world are working to develop senolytics, drugs which would clear senescent cells from the body, potentially providing treatment options for many age-related diseases that are either caused or linked to senescence. They include Alzheimer’s and Parkinson’s diseases, cardiovascular disease, osteoarthritis, macular degeneration, age-related cancers and sarcopenia, among others.
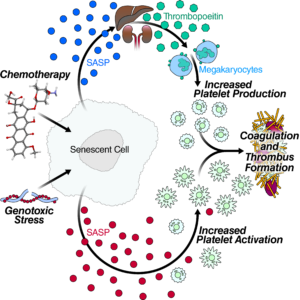 In this study, researchers validated the expression of some of the specific factors in cultured cells and in mice, which were treated with doxorubicin, a widely-used chemotherapy drug which induces widespread senescence. Those mice showed increased blood clotting, similar to what happens in humans who undergo chemotherapy. “Conversely, when we selectively removed senescent cells in specially bred transgenic mice, the increased clotting caused by doxorubicin went away,” said Christopher Wiley, PhD, assistant research professor and lead author of the paper.
In this study, researchers validated the expression of some of the specific factors in cultured cells and in mice, which were treated with doxorubicin, a widely-used chemotherapy drug which induces widespread senescence. Those mice showed increased blood clotting, similar to what happens in humans who undergo chemotherapy. “Conversely, when we selectively removed senescent cells in specially bred transgenic mice, the increased clotting caused by doxorubicin went away,” said Christopher Wiley, PhD, assistant research professor and lead author of the paper.
The way the research came together highlights the strength of the Buck’s collaborative environment says Buck professor and co-senior author Pankaj Kapahi, PhD. “We knew we had to delve into the complexity of the SASP – the senescence-associated secretory phenotype – in order understand its biological function. We brought the Schilling lab and its expertise in mass spectrometry into the mix which enabled a new understanding of how specific aspects of the SASP might be targeted. Our hope is that this research will move the field forward by improving our understanding of the implications of the SASP.”
Campisi says the work is also an endorsement of big data bioinformatics, which was used to analyze results generated in the Schilling lab. Researchers identified 343 proteins secreted by senescent human fibroblasts, with 44 linked to clotting. “Some people throw up their hands and say senescence is too complicated to make sense of research results,” Campisi said. “But this paper shows that we can identify what’s going on. That information will likely be useful in targeting who gets treatment and when they should get it.” Several labs at the Buck are involved in a federally-funded project to develop biomarkers for cellular senescence.
Citation: SILAC analysis reveals increased secretion of hemostasis-related factors by senescent cells
DOI: 10.1016/j.celrep.2019.08.049
Other Buck researchers involved in the study include Su Liu, Chandani Limbad, Anna Zawadzka, Jennifer Beck, Robert Artwood, Fatouma Alimirah, Jose-Alberto Lopez-Dominguez, Chisaka Kuehnemann, Steven R. Danielson, Natan Basisty, Herbert G. Kasler, Tal Ronnen Oron, Pierre-Yves Desprez, and Birgit Schilling. Other collaborators include Sean Mooney, Department of Biomedical Informatics & Medical Education, University of Washington, Seattle, WA; Marco Demaria, European Institute for the Biology of Aging, University of Groningen, Groningen, Netherlands; Bradford W. Gibson, Discovery Attribute Sciences, Amgen Inc., South San Francisco.
This work was funded by grants from the National Institutes of Health (F32A043252, R01AG038688, R37AG009909, and P01041122; the Larry L. Hillblom Foundation, the American Federation for Aging Research, and an NCRR shared instrumentation grant (1S10OD016281).
Judith Campisi is a founder and shareholder of Unity Biotechnology, which aims to develop senolytic drugs. The other authors declare no competing financial interests.
Science is showing that while chronological aging is inevitable, biological aging is malleable. There's a part of it that you can fight, and we are getting closer and closer to winning that fight.
Eric Verdin, MD, Buck Institute President and CEO