by Buck Institute
January 26, 2021 . Press Release
A compound that slows bone loss, and a resource for developing treatments to slow aging
A compound that extends lifespan in a tiny nematode worm slows bone loss in aging mice. That surprising result comes from a longitudinal and functional study of 700 aging mice at the Buck Institute, a project that provides a treasure trove of data for researchers aiming to develop therapeutics to slow aging and age-related diseases. The study is currently online in the Journal of Bone and Mineral Research Plus.
The project, which involved five Buck labs and took several years to complete, involved serially profiling the individual mice as they aged while testing several therapeutics that extended lifespan in simple model organisms or reduced neurological disease in mice. Researchers established rates of change for clinically significant parameters in untreated mice including kyphosis, blood glucose, body composition, activity, metabolic measures and detailed parameters of skeletal aging in bone. The study involved collecting and analyzing terabytes of data over several years.
The online application is available at https://danielevanslab.shinyapps.io/buckMouseAging/
 “This is a unique resource that comes from a study of multiple phenotypes of aging that had never been looked at before,” said Buck professor Simon Melov, PhD, senior author of the paper. “Our hope is that our data will enable those working on pre-clinical studies to essentially model experiments virtually, in order to provide a starting point for testing other interventions in mice.”
“This is a unique resource that comes from a study of multiple phenotypes of aging that had never been looked at before,” said Buck professor Simon Melov, PhD, senior author of the paper. “Our hope is that our data will enable those working on pre-clinical studies to essentially model experiments virtually, in order to provide a starting point for testing other interventions in mice.”
Benzoxazole, the compound that slowed bone aging by up to 31% over the course of a year’s treatment in the mice, was first identified as one of five compounds that extended nematode lifespan in the Lithgow lab in a study that appeared in Nature in 2011. “If you have a therapeutic that extends lifespan in a simple animal that has no bone whatsoever, you certainly wouldn’t predict that it would slow the rate of bone aging in a mammal,” said Gordon Lithgow, PhD, Buck professor and Vice President. “It’s obvious that aging-related pathways have been conserved during evolution. This new finding is a great example of the utility of screening compounds in simple animals as the starting point to look for unexpected and surprising benefits in mammals.” In the Nature article, benzoxazole appeared to suppress age-related protein aggregation. The mechanism of action in mouse bone is still under study, although researchers say the compound appears to slow the reabsorption of osteoclasts, bone cells that are active during growth and healing.
extends lifespan in a simple animal that has no bone whatsoever, you certainly wouldn’t predict that it would slow the rate of bone aging in a mammal,” said Gordon Lithgow, PhD, Buck professor and Vice President. “It’s obvious that aging-related pathways have been conserved during evolution. This new finding is a great example of the utility of screening compounds in simple animals as the starting point to look for unexpected and surprising benefits in mammals.” In the Nature article, benzoxazole appeared to suppress age-related protein aggregation. The mechanism of action in mouse bone is still under study, although researchers say the compound appears to slow the reabsorption of osteoclasts, bone cells that are active during growth and healing.
Melov says the findings in the large study are relevant to humans, especially in regards to pre-clinical phenotypes. “The metrics we used are all directly applicable to aging in humans. They literally have direct clinical correlates to the types of things you would measure in humans.” For example, for the first time researchers witnessed spontaneous fractures in aging mouse femurs. Melov says they occurred in 2.5% of the mouse population, not dissimilar to the 1 -2.7% incidence of hip fractures in people over the age of 65. He also notes that they developed a new unbiased method for evaluating kyphosis, an age-related curvature of the spine, and may pave the way for testing new interventions.
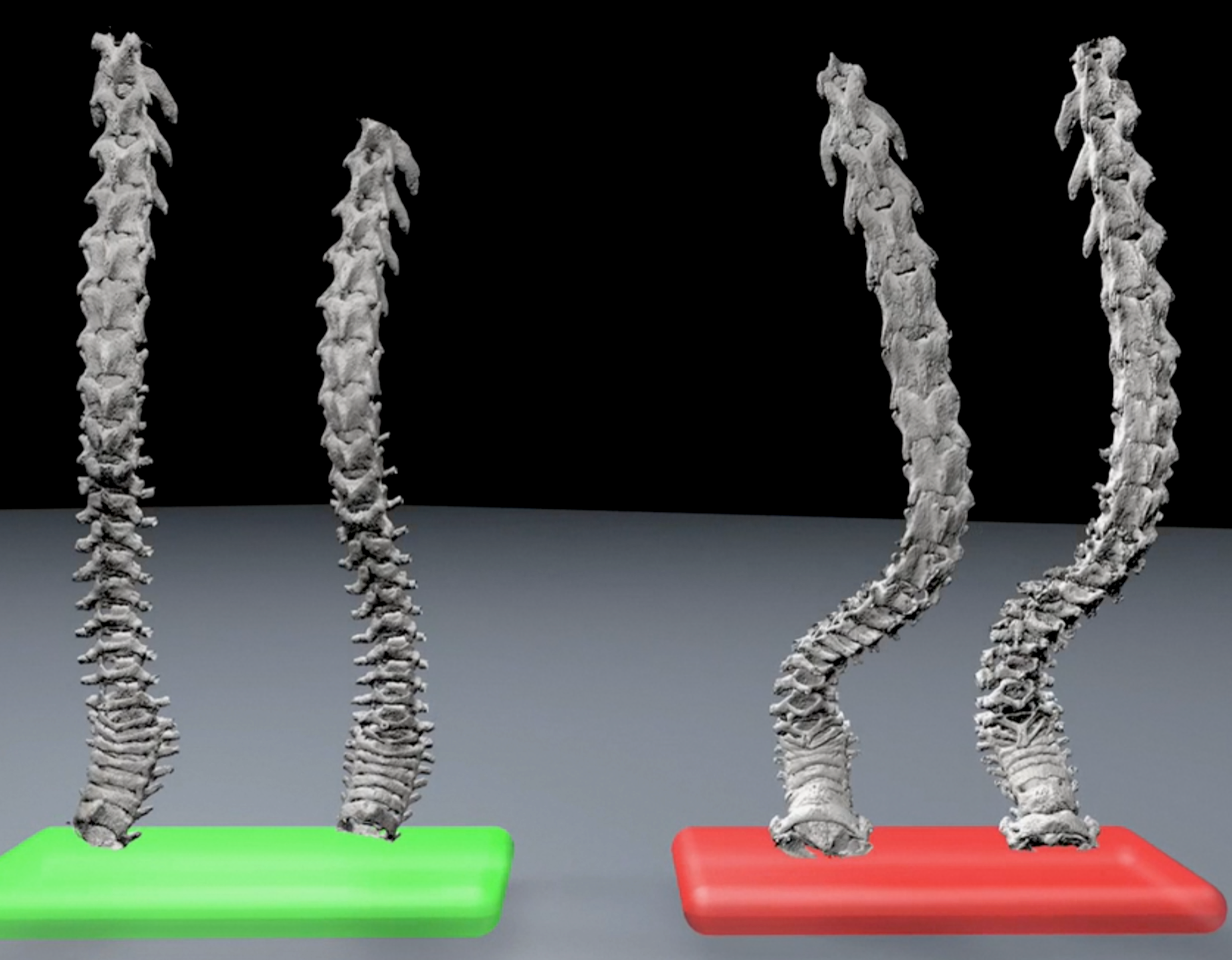
Kyphosis
Green = Young mice; Red = Old mice
“We think using this new database could save substantial resources for those wanting to do pre-clinical studies of interventions,” said Melov. “If someone wants to test a compound against a particular aging phenotype this database could provide information about how many mice are needed for the experiments and how long it would likely take to see results.”
Citation: Longitudinal Functional Study of Murine Aging: A Resource for Future Study Designs
DOI: 10.1002/jbm4.10466
Other Buck collaborators include Monique N. O’Leary, Ryan Murphy, Minna Schmidt, Kristin Koenig, Michael Presley, Brittany Garrett, Emmeline C. Academia, Matt J. Laye, Daniel Edgar, Christopher A. Zambataro, Tracey Barhydt, Colleen M. Dewey, Jarrott Mayfield, Joy Wilson, Silvestre Alavez, Brian K. Kennedy, Julie K. Andersen, Pankaj Kapahi and Gordon G. Lithgow. Other collaborators include Daniel S. Evans, California Pacific Medical Center Research Institute, Ha-Neui Kim, Li Han and Maria Almeida, University of Arkansas for Medical Sciences; and Mark Lucanic, Gerostate Alpha.
Acknowledgements
This work was supported by grants from the Larry L. Hillblom Foundation; The American Foundation for Aging Research; NIH Grants U19AG023122, U24AG051129, AG055822, AG061879, AG051129, UL1DE019608, AG038688 & AG045835, and AR56679; the Glenn Foundation for Medical Research; the Ellison Medical Foundation; and family and friends of Catherine Munson.
Science is showing that while chronological aging is inevitable, biological aging is malleable. There's a part of it that you can fight, and we are getting closer and closer to winning that fight.
Eric Verdin, MD, Buck Institute President and CEO